Chemical reaction engineering is at the heart of virtually every chemical process. All the products what we are using like waking up from foam bed, toothpaste, soap, shampoo, tea decoction, food, preserved food, glassware, LPG gas, fuel, paint, ceramic goods, agriculture needs like fertilizers, pesticides, medicines etc., all are produced from the chemical industry and not limited to above said. A chemical reaction is a process in which at least one species is transformed into chemically different species.
The subject of chemical reaction engineering initiated and evolved primarily to accomplish the task of describing how to choose, size, and determine the optimal operating conditions for a reactor whose purpose is to produce a given set of chemicals.

An adiabatic batch reactor can be defined as a closed system with no input and output streams. This batch reactor training system is operated under adiabatic conditions (Reaction Mass Temperature is not kept constant and keeps on changing), perfect mixture (composition of reaction mixture is kept uniform throughout the operation) and constant volume (volume of the reaction mixture within the reactor is kept constant hence there is no measurable change in the density of reaction mass). This training system is used to study catalytic homogeneous reaction under adiabatic condition.
The reaction is irreversible and pseudo first order with respect to acetic anhydride. The reaction is carried out adiabatically in a batch reactor. Since the reaction is exothermic, the temperature of the reactor mixture changes as the hydrolysis of acetic anhydride progresses. In order to determine the rate constant, temperature is measured at different timing.

Rate of reaction is influenced by variables like temperature, pressure, and concentration. The rate of reaction is a function of concentration at constant temperature, i.e. -rA = k CAn where n is the order of reaction. The interpretation of the kinetic data is a trial and error procedure. A kinetic model is first selected with a corresponding rate equation, and the concentration-time relationship predicted by the model is matched with experimental data. This set-up is used to study a non-catalytic homogeneous reaction under isothermal condition. Batch reactors are integral to the refining process of platinum group metals and their effective control is essential to ensure safe and efficient process in minerals industry, carbon monoxide shift conversion, ethylene oxide synthesis and synthesis of long-chain alcohols

The reaction rate constant is slightly sensitive to temperature and the values of K increases 100 times over ordinary ranges of temperature. The study of effect of temperature on K is very wide, theoretical and practical interest.

In an ideal CSTR (that is an ideal steady state flow reactor) the contents in the reactor are well mixed and have uniform composition throughout. Thus the exit stream has the same composition as the fluid within the reactor. This type of reactor is also known as Mixed Flow Reactor. Operating large reactors in industry can be expensive, so a common trick used to reduced costs is to operate multiple CSTRs in series, in which the exit stream of one CSTR is the inlet stream of the next CSTR. CSTRs are of equal volume and are operated at the same temperature, the residence time, τ will be the same for each reactor.
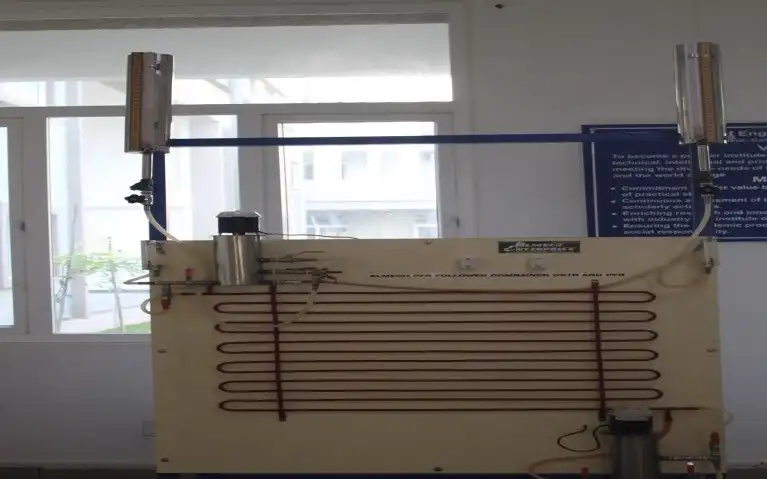
In a plug flow reactor, the composition of fluids very at different point along its length. But in mixed flow reactors compositions is almost uniform. If a plug flow and mixed flow reactors are connected in series, conversions will be high. This combination allows us to predict the overall conversions for such systems or conversions at intermediate points between the individual reactors. Finally the performance of reactors with respect to conversion has been calculated.

In a non-ideal flow the elements of the fluid taking different routes through the reactor may require different lengths of time to pass through the vessel. The distribution of these times for the stream of fluid leaving the vessel is called Residence Time Distribution (RTD) of fluid. In all experimentation, we disturb the system by injecting the tracer element and then see how the system responds to this Stimulus.
The Residence Time Distribution (RTD) method can be used for material traceability in continuous pharmaceutical tablet manufacturing process. By conducting tracer experiments using a pulse or step change of detectable material at the inlet, the response of the tracer at the outlet can be measured. The idea is that, when tablets are released, with a specific batch number and lot number, it exactly traces to what raw material batches may be present in the tablet. By changing the lot based on raw material batch composition in the tablets, it can be certain, if recall was required for specific raw material batch, which lot of tablets must be recalled as well, without recalling the entire batch, many of which tablets contain none of the recalled raw material batch. study of the hydrodynamic behaviour of a full-scale wastewater treatment plant plug-flow bioreactor.

An analysis of this Response gives desired information about the system. Initially an ideal condition is assumed according to the theory but in reality we know that any experiment that is performed should behave non-ideally. It can be proved by this experiment by comparing the Residence Time Distribution curve of the reactor with that of the ideal scenario.
The Residence Time Distribution (RTD) method can be used for material traceability in continuous pharmaceutical tablet manufacturing process. By conducting tracer experiments using a pulse or step change of detectable material at the inlet, the response of the tracer at the outlet can be measured. The idea is that, when tablets are released, with a specific batch number and lot number, it exactly traces to what raw material batches may be present in the tablet. By changing the lot based on raw material batch composition in the tablets, it can be certain, if recall was required for specific raw material batch, which lot of tablets must be recalled as well, without recalling the entire batch, many of which tablets contain none of the recalled raw material batch study of the hydrodynamic behaviour of a full-scale wastewater treatment plant plug-flow bioreactor.

Most of the chemical reactors in the industries have non-ideal regime. The non-ideal plug flow reactor (PFR) is one whose attributes deviate from that of the ideal plug flow reactors. The distribution of these times for the stream of fluid leaving the vessel is called Residence Time Distribution (RTD) of fluid. Therefore, an in-depth knowledge of the residence time distribution (RTD) of components in the reactor is necessary for its analysis.
The residence time distribution indicates how much time each fraction of a charged material spends in the vessel. The residence time distribution of reactants or tracers in a flow vessel is a key datum for determining reactor performance. The RTD is determined experimentally by injecting an inert chemical, molecule, or atom, called a tracer, into the reactor at some time t=0 and then measuring the tracer concentration, C, in the effluent stream as a function of time.

Packed bed reactors are very versatile and are used in many chemical processing applications such as absorption, distillation, stripping, separation processes, and catalytic reactions. The possible combination is any two different phases can interact with each other through solid packed material. The temperature, inlet concentration of the reactants and packing materials (arrangements) affect the conversion of reaction in packed bed reactor.
Packed tubular reactors are extensively used in chemical and associated industries such as petroleum, petrochemical, oil and gas, mineral and coal industries, pharmaceuticals, fine and specialty chemicals, and bio-chemicals. The advantage of using a packed bed reactor is the higher conversion per weight of catalyst than other catalytic reactors